



OLC
Promising innovation to address a persistent problem
Oxylanthanum Carbonate (OLC) is an unapproved investigational new drug being developed under FDA’s 505(b)(2) regulatory procedure. If approved, OLC will share substantially the same product label and prescribing information as the reference-listed drug Fosrenol® (lanthanum carbonate), with the exception that OLC tablets are smaller in size and swallowed whole with water and not chewed.
THE UNMET NEED IN HYPERPHOSPHATEMIA
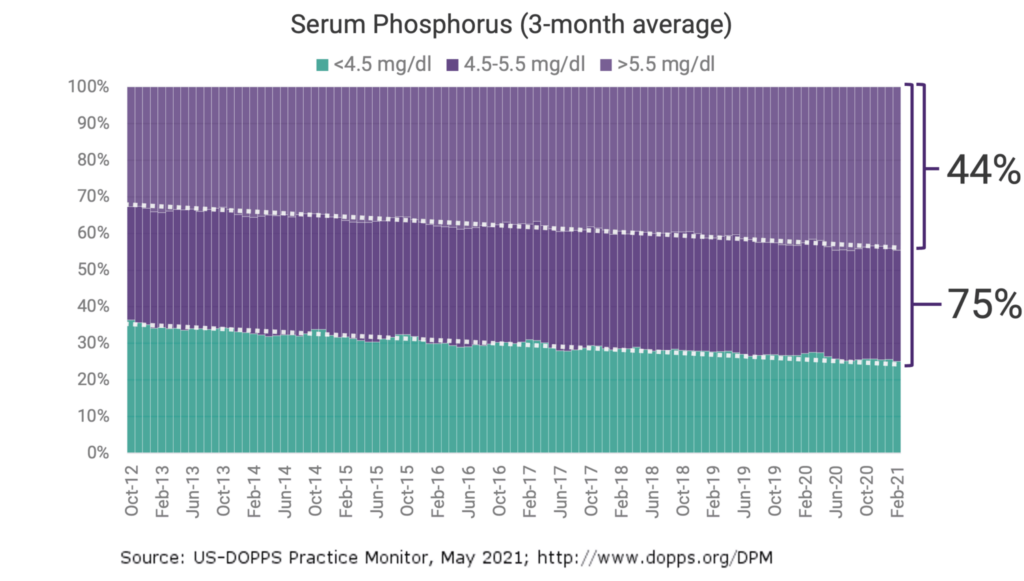
Despite the availability of 6 FDA-approved phosphate binders, hyperphosphatemia remains uncontrolled in an estimated 75% of US dialysis patients.1
Representative Sample of US Dialysis Patients
THE DAILY BURDEN FOR PATIENTS
The daily pill burden of maintenance dialysis patients is among the highest across various chronic disease states including HIV/AIDS, diabetes mellitus, and congestive heart failure.2
- 19 pills per day (median)
- 49% of pill burden from phosphate binders
- Higher pill burden was independently associated with lower quality of life scores (HR-QOL)
- 62% of patients are non-adherent (self-reported)
Unmet Needs in Treatment of Hyperphosphatemia with Phosphate Binders (Unaided)3
In a survey of 100 US-based nephrologists:
A POTENTIAL SOLUTION
Through its proprietary nanoparticle technology, UNICYCIVE has harnessed the known phosphate binding potency of elemental lanthanum to reduce the number and size of pills that patients must take to control hyperphosphatemia3*
- pill
- pill
- pill
- pill
- pill
- pill
- pill
- pill
- pill
Renvela®
sevelamer carbonate 800mg
- pill
- pill
- pill
OLC
Oxylanthanum Carbonate 1,000mg
*Average daily dose of Renvela and Fosrenol (lanthanum carbonate) from dailymed.nlm.nih.gov. Expected average daily dose of OLC based on bioequivalence to Fosrenol. Product images are proportionally sized. Fosrenol is a registered trademark of Shire International Licensing BV. Renvela® is a registered trademark of Sanofi.
References: 1. US-DOPPS Practice Monitor, May 2021; http://www.dopps.org/DPM 2. Chiu YW, Teitelbaum I, Misra M, et al. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009 Jun;4(6):1089-96. 3. Data on file. Unicycive Therapeutics, Inc; 2022.